Small batch optimization
As targeted cell and gene therapies gain more ground in today’s healthcare landscape, small-batch aseptic processing has become integral to producing these precision medicine treatments. This shift, coupled with downstream filtration challenges and a widespread push to close aseptic processes earlier in upstream development, has driven manufacturers to reevaluate how to optimize sterility throughout the process.
One of the key factors in enabling closed aseptic processes is the implementation of sterile connections – maintaining the integrity of a fluid path from start to finish – requires a focus on those points of connection between systems that may be susceptible to contamination during the joining of two systems. Traditionally, in small batch applications, these connections have been supported through manual, open processes such as quick connects and luers, which require connections to be completed under laminar flow hoods, or tube welding, which uses heat to fuse open tubing ends together to create a sterile connection. Tube welding requires its own dedicated equipment and power source, which can impose space and time constraints that become more cumbersome as manufacturers ramp up production. While traditional biopharma applications have adopted more processes centered around closing of systems utilizing sterile connectors, the cell therapy and gene therapy have been slower to adoption of those processes. As these modalities remain relatively new and less known to some, many cell and gene therapy processes still employ tube welding or open connections; not yet adopting single-use aseptic connections.
While sterile aseptic connectors have been widely adopted in larger aseptic processing applications, ultra-compact sterile connectors were not available for closed small volume (<10L) processes until recently. Their use in small-batch processes has begun to experience more widespread adoption, largely due to the time and cost savings these connectors represent: as autologous cell therapies and other targeted gene therapies work to scale up to commercial levels, connections that can be changed out in seconds, rather than in the minutes typical of traditional connection processes, represent a distinct advantage for industry. Their ease-of-use, one time validation, and flexibility in integrating with existing processes make single-use aseptic connectors ideal for use in challenging bioprocessing and personalized medicine conditions.
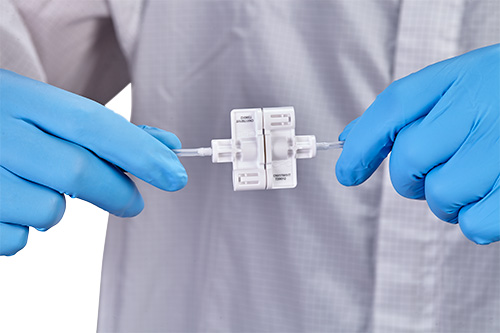 1/16" tubing with sterile connector
1/16" tubing with sterile connector
Integrating Sterile Mini-Connectors for Small-Scale Cell and Gene Processes
Sterile connectors are designed to link one sterile fluid stream to another – from a container to a sampling line, media to a product vessel, or a filtration assembly to a filling line. These connectors offer a number of advantages over traditional modalities. Workforce turnover and constraints, pressures surrounding speed to market, increased competition – challenges inherent to even small-scale manufacturing operations —necessitate faster, more streamlined production environments. Surmounting these challenges is especially critical in the cell and gene therapy space, which often utilizes an individual patient’s cells or DNA to produce individualized treatments.
Preventing contamination can be a costly and labor-intensive endeavor. Additionally, the filtration steps used in other biopharmaceutical manufacturing cannot translate to cell therapy, where the cells in question are filtered out alongside any contaminants. Finding approaches that reduce the risk of contamination for these complex therapies in ways that are transferrable, scalable, and effective is essential.
Tube welding, a common means of establishing a sterile connection within a closed system, requires the use of pressure and heat to connect two segments of tubing. While this approach has been utilized for decades to enable closed processing, there are potential drawbacks that may compromise the sterility of an application. If the weld is performed improperly, this introduces the risk of occlusion, or a partial obstruction of the flow path that occurs during the sealing process. Shifting to a robust, validated, easier-to-use process incorporating aseptic connectors rather than tube welding in small-scale processing represents an opportunity to improve production yields, decrease time-to-market, and reduce costs.
 Sterile barrier for more protection during transfer
Sterile barrier for more protection during transfer
Facilitating the shorter production runs and increased changeover driving cell and gene production requires new solutions built for speed. As cell therapy manufacturing facilities move closer to patients, agility, quick turn-around process while maintaining sterility has been a priority by companies that are manufacturing in this space. In addition, more biopharmaceutical companies are reaping the benefits of closing their processes as early in the development pipeline as possible and utilizing sterile connectors, which are available in a range of sizes, simplifying scalability.
Streamlining and Accelerating Aseptic Processing with Single-Use Technologies
Single-use bioprocessing is designed to be flexible, efficient, and effective in the manufacture of drug substances, monoclonal antibodies, vaccines, biosimilars, cell and gene therapies, and other modalities. Genderless, easy-to-use single-use connectors are a key tool in enabling this paradigm. By reducing system complexity, simplifying training requirements, and enabling rapid process conversion, these products help manufacturers accelerate their timelines.
In contrast to both tube welding and laminar flow hood methodologies, connectors do not require any additional equipment or capital investment in order to integrate them into existing processes. The training needed to incorporate connectors in a system is minimal, as is the time needed to change connectors – roughly 15 seconds, compared to tube welding, which can take more than a minute for a trained operator to complete. This time savings is another factor that, when applied to thousands of changeovers, creates attractive economies of scale for manufacturers.
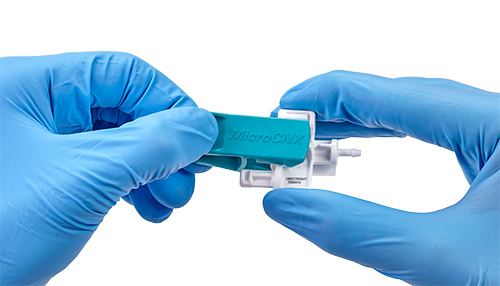
Until recently, tube welding was the only option for creating closed aseptic systems at small volumes. This lack of very small aseptic connector options led to the development of a first-of-its-kind connector designed specifically for small-format assemblies (MicroCNX® Series Connectors, CPC, St. Paul, Minn.). This small-volume connector features simplified “pinch-click-pull” installation: users pinch to remove the connector’s protective cover, click its two halves together, and pull out the protective membranes to allow flow to move through the connector.
Quick and easy assembly
This process offers a stark contrast to tube welding, which requires at least a dozen steps, access to electricity, routine equipment maintenance, and comparatively extensive training. It also allows manufacturers to maximize their cleanroom space by eliminating the need for multiple welders and the space required to maneuver them. The MicroCNX connectors’ intuitive, simplified connection process also helps reduce the risk of operator error, minimizing the related operator performance and reliability concerns. CPC, a global leader in single-use connection technology, has pioneered the MicroCNX series in response to a burgeoning need for more reliable aseptic connection techniques in small-volume processing.
Ultimately, single-use aseptic micro-connectors represent an efficient, effective method for closing small-scale manufacturing systems quickly and reliably. Their implementation in smaller production paradigms has the potential to streamline scale up, shortening lead times and enabling greater speed-to-market through subsequent capacity expansion and the optimal use of high-value production space.